Abstract
Background: Malignant lymphoid cells are characteristically dependent on signals mediated by the phosphatidylinositol 3-kinase delta isoform (PI3Kδ). Idelalisib is an oral, selective, PI3Kδ inhibitor approved by the FDA and EMA as monotherapy for the treatment of advanced follicular lymphoma (FL). The approval was based on a phase 2 trial in patients with indolent non-Hodgkin lymphoma including 72 patients with FL who were refractory to at least 2 prior regimens (NCT01282424; Study 101-09). Interim safety analysis of this large, pan-European, noninterventional study of refractory FL patients treated with idelalisib monotherapy (EUPAS19618; NCT03568929) showed that the adverse event (AE) profile in clinical practice corroborates the known safety profile of idelalisib reported from clinical trials. Herein, we report the effectiveness and updated safety analysis from this study.
Methods: This was a non-interventional, retrospective, cohort study. Adult patients treated with idelalisib for FL in routine clinical practice in 10 European countries were included. Data were collected retrospectively from sites by study personnel from remotely source data-verified medical records using electronic case report forms. Safety and effectiveness data for each patient were collected from time of idelalisib initiation until 6 months post-discontinuation of idelalisib, start of next treatment, or death. For this analysis, the data cut-off date was 16 June 2021. Effectiveness of idelalisib was assessed by overall response rate (ORR), duration of response (DOR), progression-free survival (PFS), time to next treatment (TTNT), and overall survival (OS). The overall safety profile of idelalisib was assessed by estimating the incidence of AEs, serious AEs, adverse drug reactions (ADRs), and serious ADRs. Focus was given to special health outcomes of interest (HOIs), including transaminase elevation, hepatocellular injury, severe diarrhea/colitis, pneumonitis, neutropenia, rash, Stevens-Johnson syndrome, and serious infections. Multivariate Poisson regression analyses are used to estimate rates of ADRs, serious ADRs, and HOIs, and are adjusted for potential confounders. Time-to-event data were analyzed using Kaplan-Meier methods.
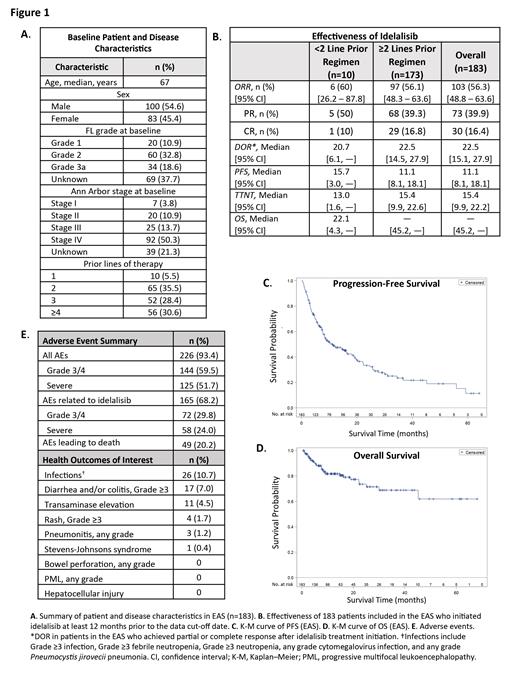
Results: Overall, there were 257 eligible patients in the study from 85 sites. The Full Analysis Set (FAS) consisted of 242 patients excluding those with deviations (n=10) or those without any data contribution at the cut-off date (n=5). Of the 242 patients, 183 initiated idelalisib at least 12 months prior to data cut-off date and were included in the Effectiveness Analysis Set (EAS; n=49 patients did not have effectiveness data recorded, and 10 did not meet effectiveness inclusion criteria). For the 183 patients in the EAS, median age was 67 years and 54.6% (n=100) were male (Fig. 1A). Median number of prior therapies was 3 (range 1-10) and median time since diagnosis was 5.8 years (range: 0.4-32). At treatment initiation, 64% (n=117) had Ann Arbor stage III/IV disease. Median duration of idelalisib exposure was 6.8 months (IQR 3.0-15.6); 41.5% (n=76) had dose interruptions. Patients received doses of 150 mg only (67.8%), both 150 mg and 100 mg (28.4%), or 100 mg only (3.8%). Patients were observed for a median of 9.8 months (IQR 5.2-19.3). In the EAS, 103/183 patients achieved a response with 30 patients (16.4%) achieving a complete response (CR) and 73 (39.9%) achieving a partial response (PR), yielding a best ORR of 56.3% (95% CI: 48.8-63.6; Fig. 1B). Median DOR, PFS, and TTNT were 22.5 (95% CI: 15.1-27.9), 11.1 (95% CI: 8.1-18.1) and 15.4 months (95% CI: 9.9-22.2), respectively (Fig. 1B, C). Median OS had not been reached at the time of data cut-off (Fig. 1D). Updated safety information was available from the 242 patients in the FAS. The most frequent AEs recorded were infections, diarrhea and/or colitis, and transaminase elevation. The proportion of patients in the FAS experiencing Grade 3/4, severe, common, and HOI TEAEs are presented in Fig. 1E.
Conclusions: We report to our knowledge the largest cohort of FL patients treated with idelalisib outside of the clinical trial setting. Our effectiveness findings are remarkably similar to those from the registrational study 101-09 (Gopal 2014 N Engl J Med). Safety profile of idelalisib was consistent with trial experience, and no new safety signals were identified. Idelalisib remains an effective treatment option for FL patients.
Gyan: Gilead Sciences, Inc.: Consultancy; Novartis: Research Funding; Mundipharma: Research Funding; Fresenius Kabi: Research Funding; Sanofi: Honoraria; AbbVie: Other: Hospitality; AstraZeneca: Honoraria. Tisi: Incyte: Membership on an entity's Board of Directors or advisory committees; Bristol Myers Squibb: Membership on an entity's Board of Directors or advisory committees; Novartis: Membership on an entity's Board of Directors or advisory committees; Gilead Sciences, Inc.: Honoraria, Membership on an entity's Board of Directors or advisory committees. Merli: Takeda: Consultancy; Janssen Pharmaceuticals: Consultancy; Gilead Sciences, Inc.: Consultancy; Novartis: Consultancy. Di Raimondo: Amgen: Honoraria; AbbVie: Honoraria; Pfizer: Honoraria; Bristol Myers Squibb: Honoraria; Janssen Pharmaceuticals: Honoraria; Jazz Pharmaceutical: Honoraria. Mercadal: Gilead Sciences, Inc.: Honoraria, Speakers Bureau; Roche: Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau. Puccini: Takeda: Membership on an entity's Board of Directors or advisory committees. Vandenberghe: Janssen Pharmaceuticals: Honoraria; AbbVie: Honoraria. Boland: Gilead Sciences, Inc.: Current Employment, Current equity holder in publicly-traded company. Shah Gupta: Gilead Sciences, Inc.: Current Employment, Current equity holder in publicly-traded company. van Troostenburg: Gilead Sciences, Inc.: Current Employment, Current equity holder in publicly-traded company. Dunnill: Gilead Sciences, Inc.: Current equity holder in publicly-traded company, Other: Contractor. Ramroth: Gilead Sciences, Inc.: Current Employment, Current equity holder in publicly-traded company. Rajakumaraswamy: Gilead Sciences, Inc.: Current Employment, Current equity holder in publicly-traded company. Salles: Genentech/Roche: Consultancy; Incyte: Consultancy; Regeneron: Consultancy, Honoraria; Miltneiy: Consultancy; Genmab: Consultancy; Epizyme: Consultancy, Honoraria; Velosbio: Consultancy; Novartis: Consultancy; Ipsen: Consultancy; Morphosys: Consultancy, Honoraria; Allogene: Consultancy; Debiopharm: Consultancy; Janssen: Consultancy; Kite/Gilead: Consultancy; Loxo: Consultancy; Takeda: Consultancy; Rapt: Consultancy; BMS/Celgene: Consultancy; Beigene: Consultancy; Abbvie: Consultancy, Honoraria; Bayer: Honoraria.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal